Non Ce Marked Medical Devices . Mdr article 2(45) defines ‘clinical investigation’ as: Only ce marked medical devices may be promoted and placed on. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. the medical device directive 93/42/eec provides the following basic provisions: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. ‘any systematic investigation involving one or. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise.
from www.ce-marking.com
amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. ‘any systematic investigation involving one or. the medical device directive 93/42/eec provides the following basic provisions: Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical investigation’ as:
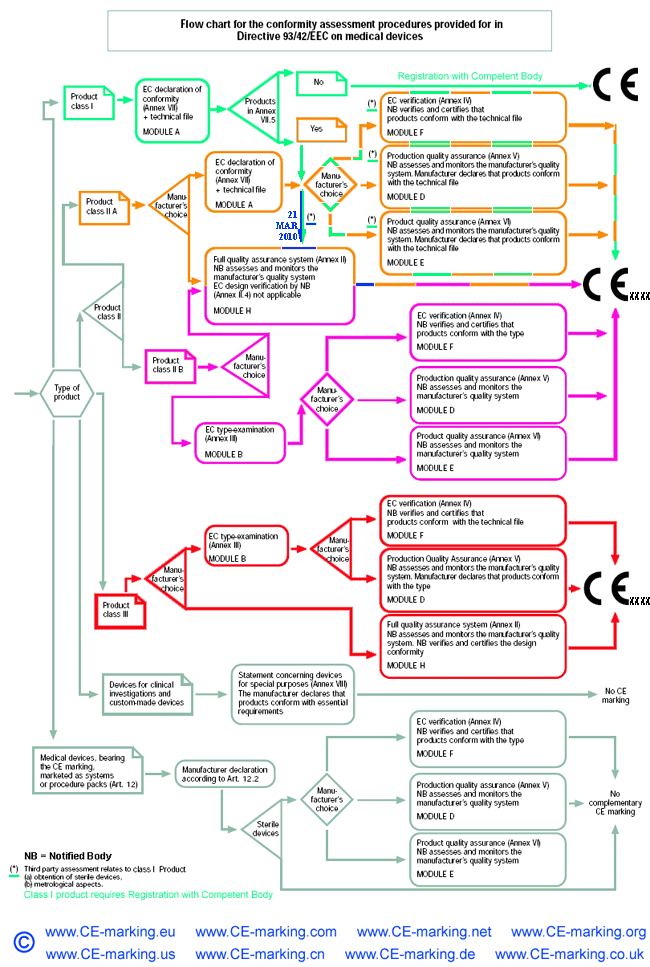
List of CE marking conformity modules applicable for Medical Device (MD
Non Ce Marked Medical Devices amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. the medical device directive 93/42/eec provides the following basic provisions: ‘any systematic investigation involving one or. amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical investigation’ as: The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise.
From www.linkedin.com
Get your Medical devices CE Marked Non Ce Marked Medical Devices the medical device directive 93/42/eec provides the following basic provisions: Mdr article 2(45) defines ‘clinical investigation’ as: ‘any systematic investigation involving one or. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. amendment to article 59 of the eu mdr allows for the sale and distribution of medical. Non Ce Marked Medical Devices.
From www.rapidlabs.co.uk
AntiA, AntiB, AntiA,B & AntiD Kit CE Marked Rapid Labs Non Ce Marked Medical Devices Mdr article 2(45) defines ‘clinical investigation’ as: the medical device directive 93/42/eec provides the following basic provisions: Only ce marked medical devices may be promoted and placed on. amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. The purpose of this document. Non Ce Marked Medical Devices.
From www.clinicalservicesjournal.com
Timeframe for accepting CE marked medical devices in Great Britain extended Non Ce Marked Medical Devices Mdr article 2(45) defines ‘clinical investigation’ as: ‘any systematic investigation involving one or. the medical device directive 93/42/eec provides the following basic provisions: a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that. Non Ce Marked Medical Devices.
From www.indiamart.com
Medical Devices CE Marking Certification Service in Dadumajra, Eucert Non Ce Marked Medical Devices The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Mdr article 2(45) defines ‘clinical investigation’ as: ‘any systematic investigation involving one or. amendment to article 59 of the eu mdr allows. Non Ce Marked Medical Devices.
From clin-r.com
Labels for Medical Devices Clin R Non Ce Marked Medical Devices The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. Only ce marked medical devices may be promoted and placed on. the medical device directive 93/42/eec provides the following basic provisions: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that. Non Ce Marked Medical Devices.
From angelanjohnson.com
Medical Devices Angela N Johnson Non Ce Marked Medical Devices a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical. Non Ce Marked Medical Devices.
From www.i3cglobal.com
CE Marking Approval For Medical Device I3CGlobal Non Ce Marked Medical Devices amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. ‘any systematic investigation involving one or. Only ce marked medical devices may be promoted and placed on. the medical device directive 93/42/eec provides the following basic provisions: Mdr article 2(45) defines ‘clinical investigation’. Non Ce Marked Medical Devices.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Non Ce Marked Medical Devices amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical investigation’ as: the medical device directive 93/42/eec provides the following basic provisions: a medical device may. Non Ce Marked Medical Devices.
From www.pathologyinpractice.com
Extended acceptance of CEmarked medical devices Non Ce Marked Medical Devices Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical investigation’ as: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. a medical device may contain an ancillary medicinal substance to support the proper functioning of. Non Ce Marked Medical Devices.
From www.gov.uk
Medical devices extended acceptance of CE marked medical devices on Non Ce Marked Medical Devices ‘any systematic investigation involving one or. the medical device directive 93/42/eec provides the following basic provisions: Mdr article 2(45) defines ‘clinical investigation’ as: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. Only ce marked medical devices may be promoted and placed. Non Ce Marked Medical Devices.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Non Ce Marked Medical Devices the medical device directive 93/42/eec provides the following basic provisions: a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. ‘any systematic investigation involving one or. Only ce marked medical devices may be promoted and placed on. Mdr article 2(45) defines ‘clinical investigation’ as: amendment to article 59 of the. Non Ce Marked Medical Devices.
From 3dprintingindustry.com
WiDE 3D printed prosthetic customization receives CE marking 3D Non Ce Marked Medical Devices amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Mdr article 2(45) defines ‘clinical investigation’ as: ‘any systematic investigation involving one or. Only ce marked. Non Ce Marked Medical Devices.
From es.slideshare.net
Regulation of Medical Devices in US Non Ce Marked Medical Devices amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. ‘any systematic investigation involving one or. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. Only ce marked medical devices may be promoted and. Non Ce Marked Medical Devices.
From www.linkedin.com
MDSS on LinkedIn Timelines for CE Marked Medical Device Placement in Non Ce Marked Medical Devices Mdr article 2(45) defines ‘clinical investigation’ as: The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. ‘any systematic investigation involving one or. Only ce marked medical devices may be promoted and placed on. a medical device may contain an ancillary medicinal substance to support the proper functioning of the. Non Ce Marked Medical Devices.
From www.ce-marking.com
List of CE marking conformity modules applicable for Medical Device (MD Non Ce Marked Medical Devices ‘any systematic investigation involving one or. amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. The purpose of this document is to help clinical investigators by highlighting a number of specific requirements that arise. the medical device directive 93/42/eec provides the following. Non Ce Marked Medical Devices.
From medium.com
The Definitive Guide to CE Marking for Medical Devices by Non Ce Marked Medical Devices Only ce marked medical devices may be promoted and placed on. a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. ‘any systematic investigation involving one or. amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without. Non Ce Marked Medical Devices.
From www.youtube.com
7 Steps How to Get a CE Marking Certification for Medical Devices Non Ce Marked Medical Devices the medical device directive 93/42/eec provides the following basic provisions: a medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Mdr article 2(45) defines ‘clinical investigation’ as: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health. Non Ce Marked Medical Devices.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Non Ce Marked Medical Devices Only ce marked medical devices may be promoted and placed on. the medical device directive 93/42/eec provides the following basic provisions: Mdr article 2(45) defines ‘clinical investigation’ as: amendment to article 59 of the eu mdr allows for the sale and distribution of medical devices that are essential for public health without a. a medical device may. Non Ce Marked Medical Devices.